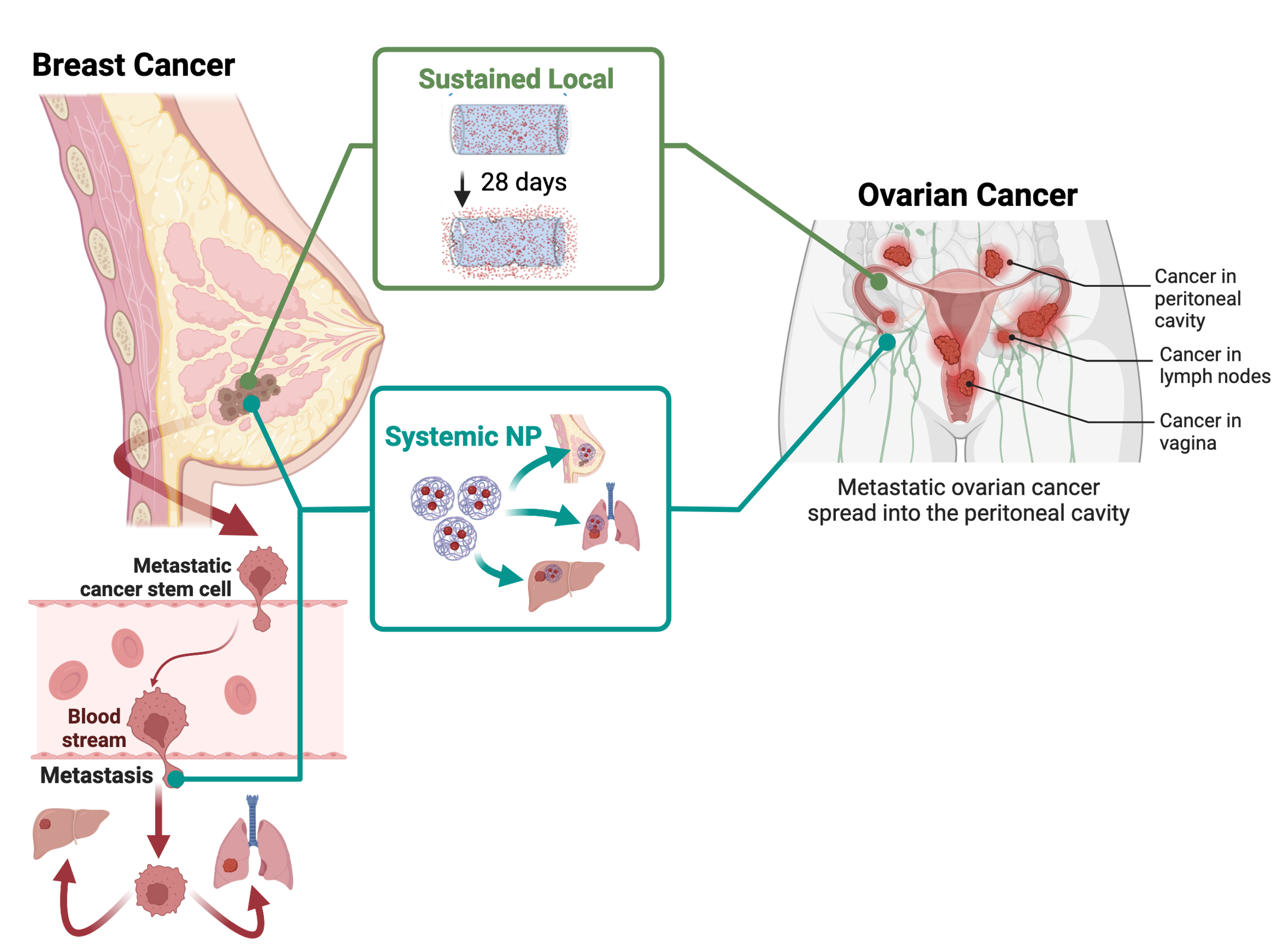
Therapies that enter clinical investigation teeter the line between toxic and effective dose. In an ideal situation, the effective dose is much lower than the toxic dose, however in most clinical trials we discover the inverse is oftentimes true leading to a high failure rate of 90%. There is an unmet clinical need to develop strategies that bridge the gap between preclinical and clinical translation of novel therapies to maximize efficacy and minimize toxicity. Our goal is to expand the arsenal of nanomaterial designs to circumvent these barriers to drug delivery.
Check out this short video about our work: (CECS UCF Seminar: Nanomaterials for biomedical applications)
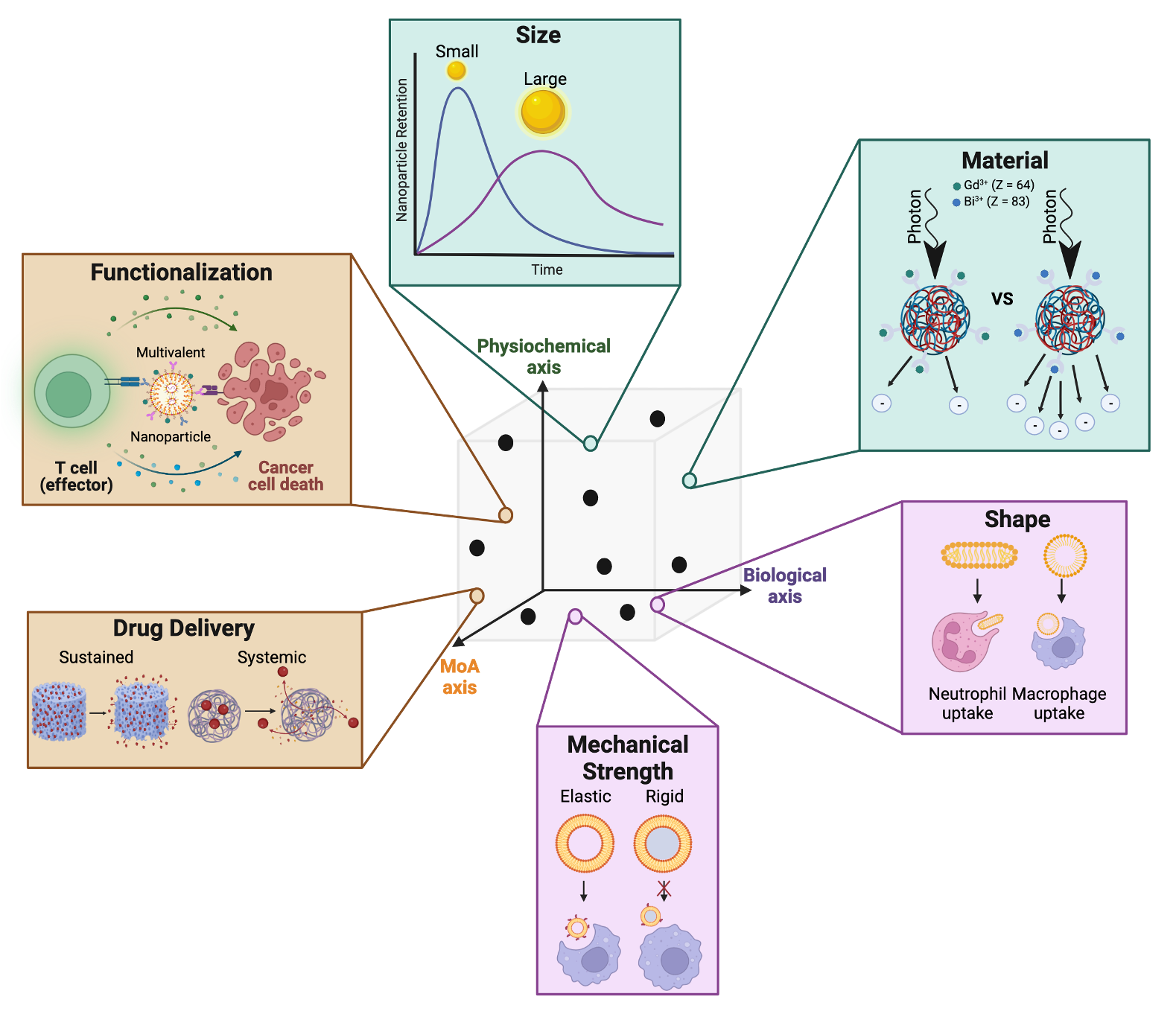
A critical challenge with nanoparticle translation today is the inability to control toxic off-target accumulation. In a recent literature survey, it was found that on average only 0.7% of the administered nanoparticle is delivered to the tumor and this number has largely remained constant over the last decade. The remaining 99.3% are either cleared via the renal system or taken up by macrophages. Fighting the biology is an uphill battle, instead can we design nanoparticles to leverage these biological interactions?
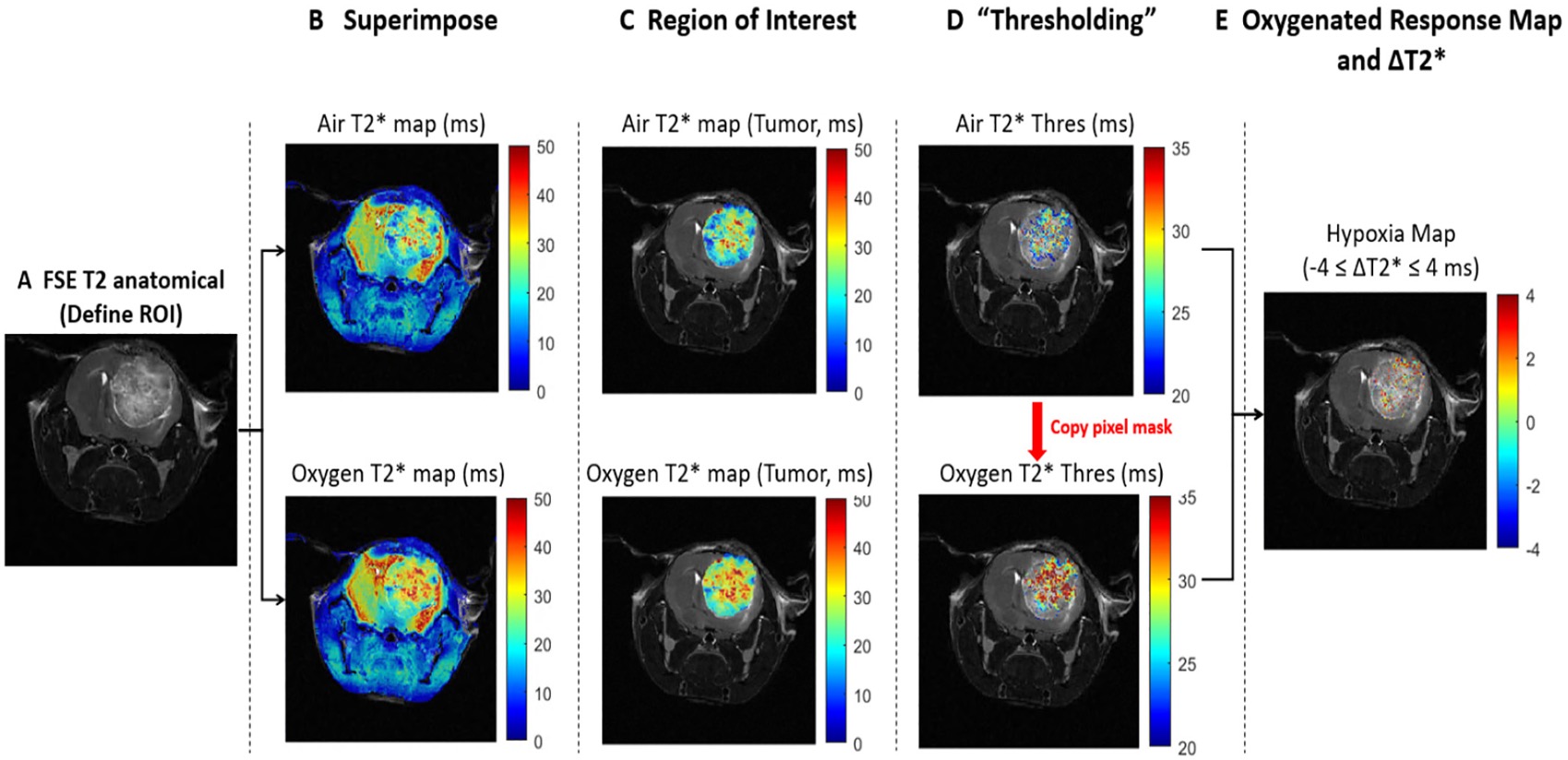
Therapies will only be effective if we are able to prescribe it to the right patient at the right time. Currently, efficacy of treatment is monitored by waiting months to see if a patient responds, however for nonresponsive patients this allows time for metastases and drug resistance to develop. Building tools to assess response early during the treatment period and stratify tumor composition in real time will allow clinicians to better treat patients as well as improve response for our nanotherapeutics.